History
GLADEL has more than 20 years of sustained work with a significant number of contributions to the field of lupus, not only in the differential role that race/ethnicity plays in its course and outcome but also in several other studies including the beneficial effects of using antimalarials in lupus patients and the development of consensus guidelines for the treatment of lupus in our region.
Objective
A new generation of “Lupus Investigators” throughout Latin America launched a novel study that aim to identify subgroups of patients with SLE, based on demographic and clinical profile, serum and urinary biomarkers and transcriptome studies using blood and tissue RNA to identify potential transcriptional signatures.
This study is supported and funded by Janssen Research & Development, LLC.
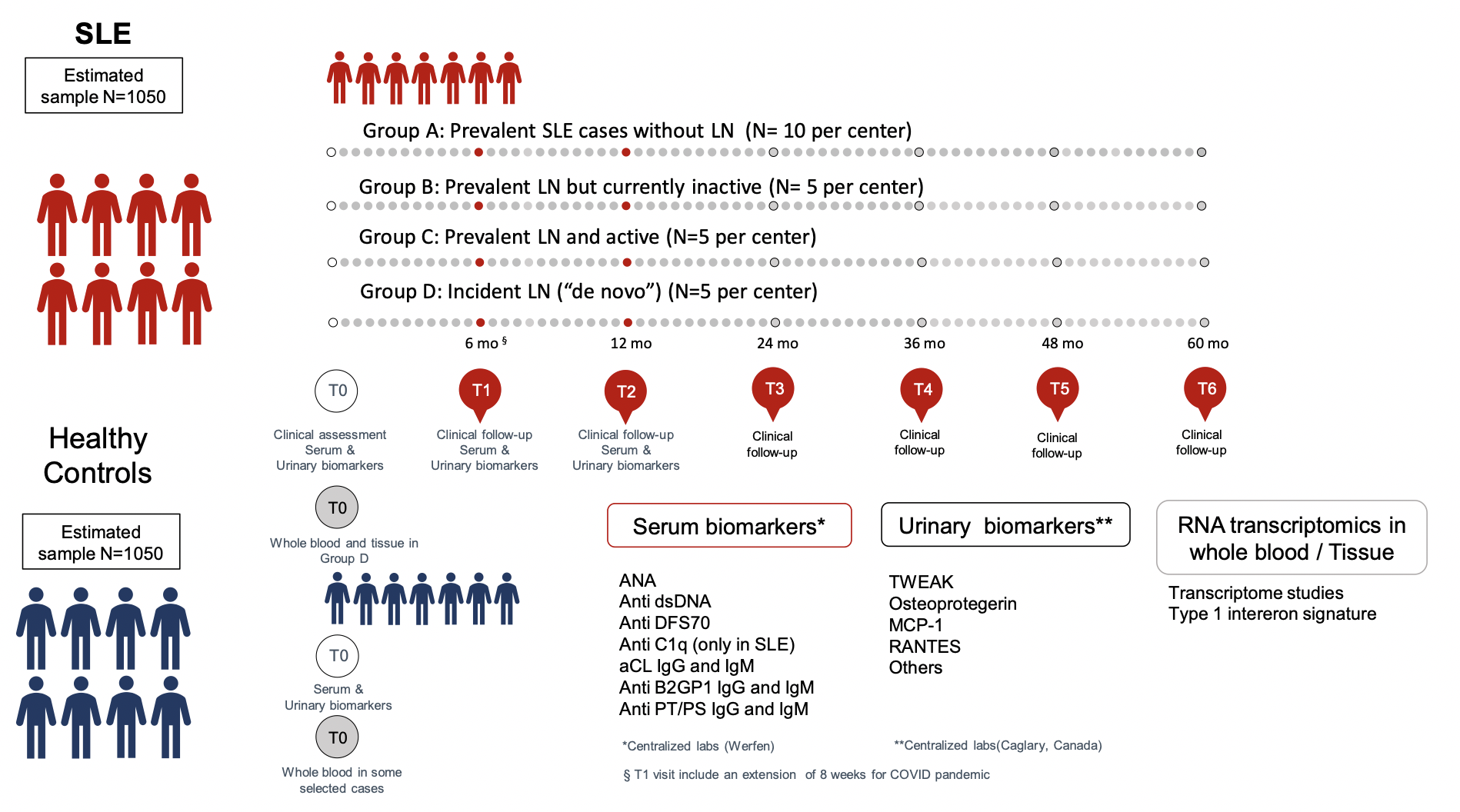
GLADEL 2.0 Cohort. Study design.
Latin-American registry.
- ~150 investigators
- 42 centers
- 10 countries
- 2,100 individuals
- 1,050 SLE patients
- 1,050 controls (healthy non-relative control at ratio 1:1, matched by age (± 5 years), sex and ethnic group)
- +500 variables (sociodemographic, clinical and patients reported outcomes)
- Centralize analysis of:
- Serological biomarkers
- Urinary biomarkers
- Transcriptome
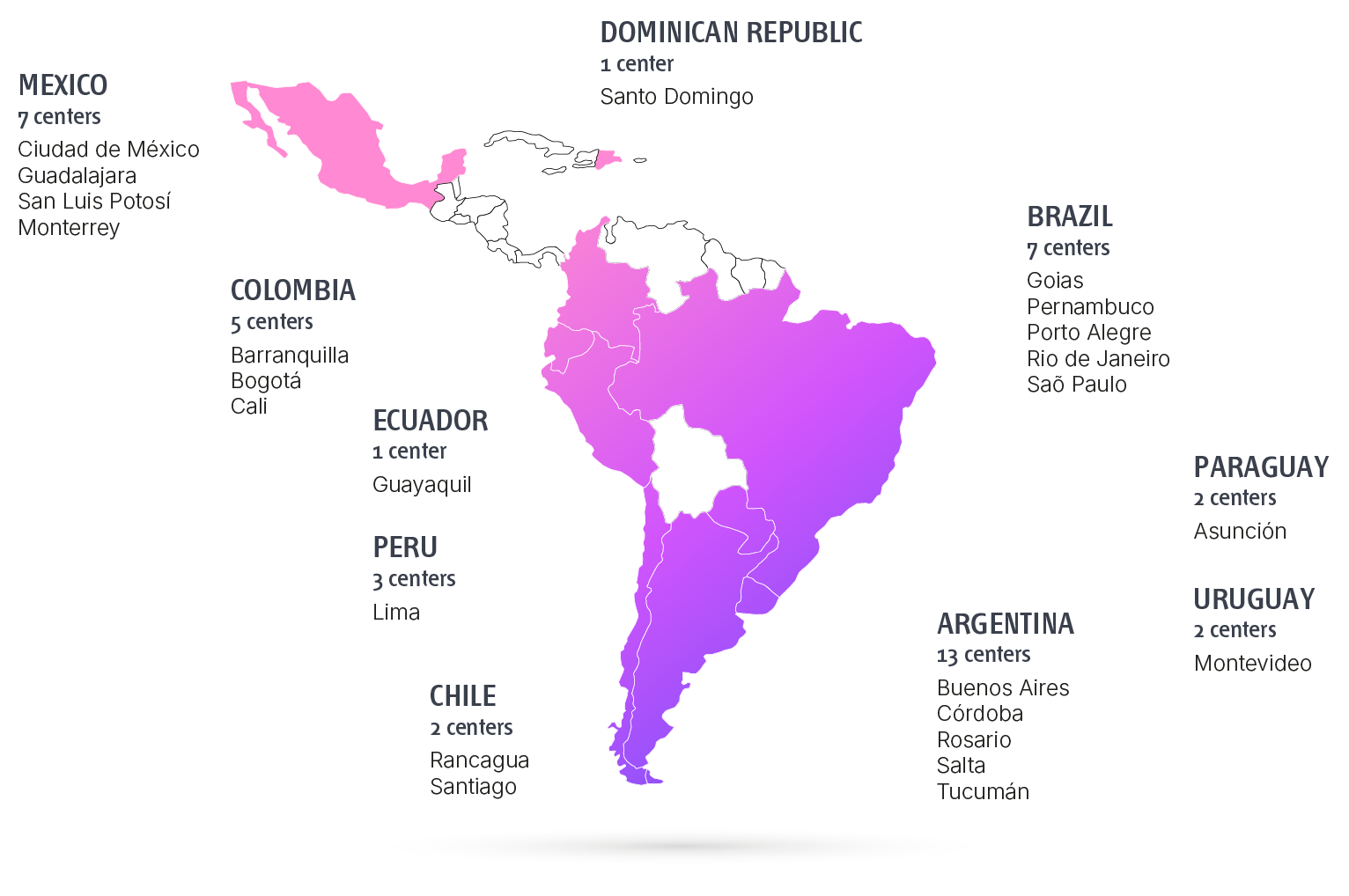
Research Centers
ARGENTINA
- Hospital Italiano de Buenos Aires, Buenos Aires
- Hospital Italiano de Córdoba, Córdoba
- Hospital Córdoba, Córdoba
- Hospital Privado Universitario de Córdoba, Córdoba
- Hospital HIGA San Martín, La Plata
- Sanatorio Británico, Rosario
- Sanatorio Parque, Rosario
- Hospital Padilla, Tucumán
- Hospital General de Agudos J.M. Ramos Mejía, Buenos Aires
- Instituto de Investigaciones Médicas Alfredo Lanari, Buenos Aires
- CEMIC, Buenos Aires Hospital Señor del Milagro, Salta
BRASIL
- Universidade Estadual de Campinas, Faculdade de Ciencias Médicas
- Hospital das Clinicas, Univerisad Federal de Goias, Goias
- Hospital de Clínicas de Porto Alegre, Porto Alegre
- Universidad Federal de Pernambuco, Pernambuco
- Hospital Universitario Pedro Ernesto, UERJ, Rio de Janeiro
- Hospital Das Clinicas da Faculdade de Medicina da USP, São Paulo
- Universidad Federal São Paulo, São Paulo C
CHILE
- Hospital del Salvador, Santiago
- Facultad de Medicina, Pontificia Universidad Catolica de Chile, Santiago
- Universidad San Sebastián, Rancagua.
COLOMBIA
- Clínica de la Costa Ltda., Barranquilla
- Fundación Valle del Lili, Cali Universidad de Antioquia, Medellín
- Fundación Santa Fe, Bogotá
ECUADOR
- Hospital Luis Vernaza, Guayaquil
MÉXICO
- Centro Médico La Raza, Ciudad de México
- Centro Médico Nacional Siglo XXI, Ciudad de México
- Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Ciudad de México
- Instituto Nacional de Cardiología Ignacio Chávez, Ciudad de México
- Centro de Estudios de investigación Básica y Clínica S.C, Guadalajara
- Hospital Central Dr. Ignacio Morones Prieto, San Luis Potosi
- Hospital Universitario “Dr. José Eleuterio Gonzalez", Monterrey
PARAGUAY
- Hospital de Clínicas I, Asunción
- Hospital de Clínicas II, Asunción
PERÚ
- Hospital Nacional Edgardo Rebagliatti Martins, Lima
- Hospital Nacional Guillermo Almenara Irigoyen, Lima
- Hospital Cayetano Heredia, Lima
REPUBLICA DOMINICANA
- Hospital Docente Padre Bellini, Santo Domingo
URUGUAY
- Clínica Médica C, Hospital de Clínicas, UDELAR, Montevideo
- Grupo de Investigación de EAIS y Reumatológicas, Montevideo